One alternative to the chemical battery for storing electrical energy is the supercapacitor. Also known as the Electric Double-Layer Capacitor (EDLC), supercapacitors are built from electrodes coated in a porous material, which is usually carbon-based, separated by an electrolyte that is itself divided by a membrane. A typical supercapacitor structure is shown in the figure below.
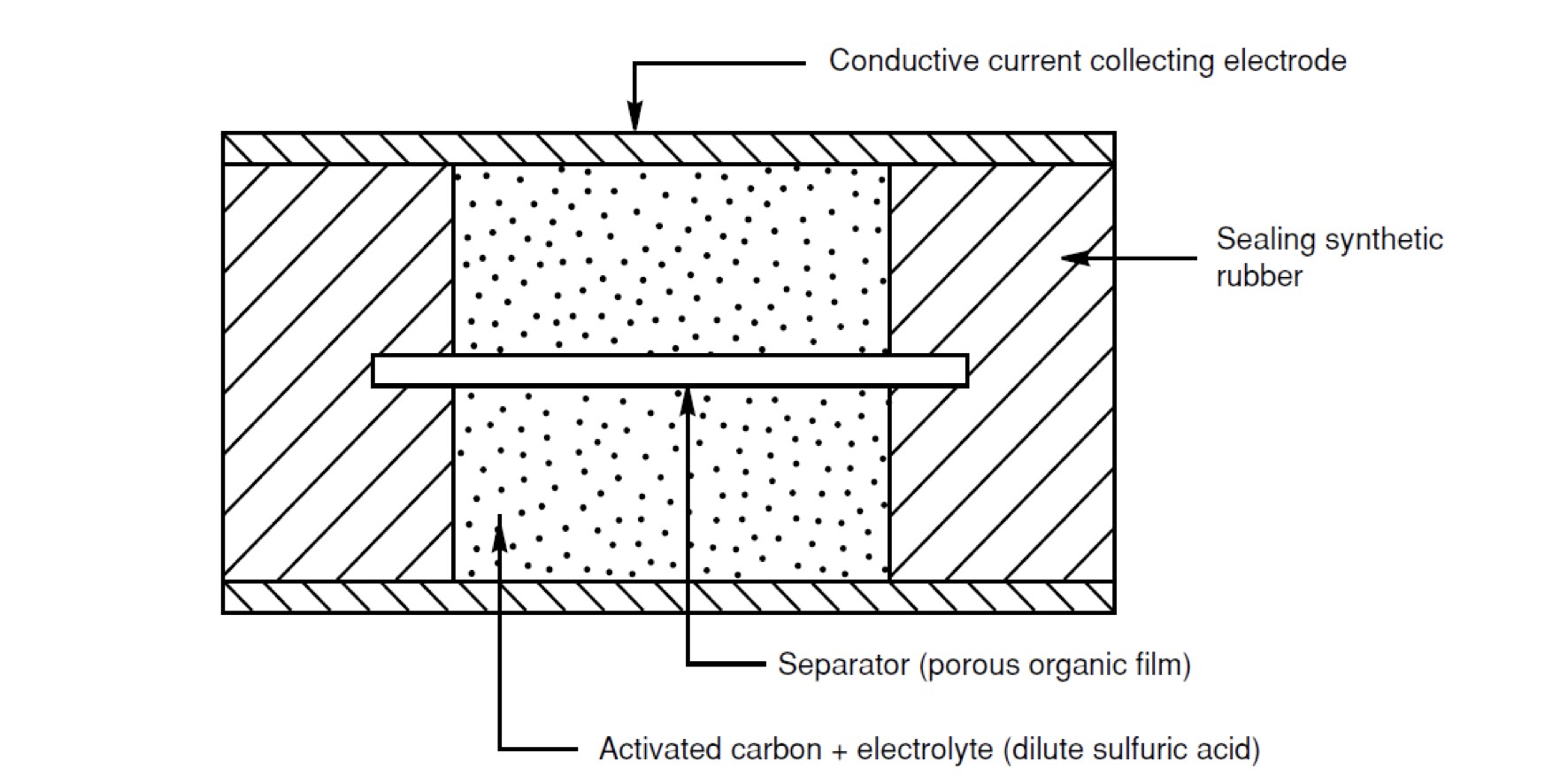
Figure 1: Basic supercapacitor structure
Unlike a battery, the supercapacitor stores and releases energy quickly through physical adsorption and ions’ desorption in the electrolyte between its electrodes. When two different solid and liquid phases come into contact, positive and negative charges are distributed at the interface across a small distance. The charge layer along this boundary surface is called the “electrical double layer.” The figure below shows the double layer and its primary function to store energy like a capacitor.
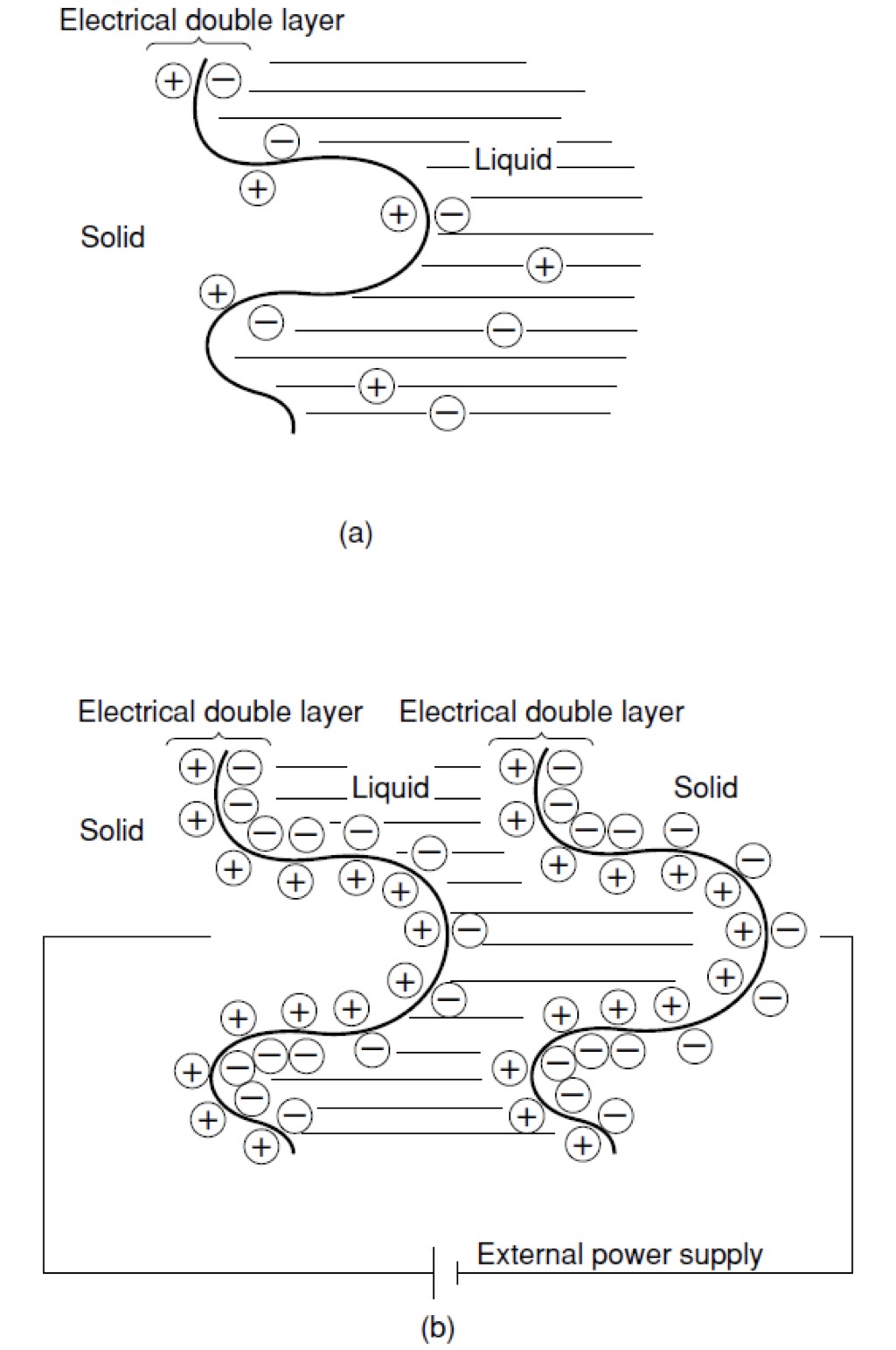
Figure 2: (a) Electrical double layer and (b) capacitor with applied external voltage
Modern advances in carbon-based materials enable porous electrodes to have a large surface area resulting in a high capacitance density and small physical form factor. Such high volumetric efficiency makes them an ideal candidate for replacing small secondary coin cell batteries. The ionic processes used to store energy in supercapacitors are also relatively fast. The device can fully charge within a few seconds, whereas a typical battery cell can take from ten minutes to several hours to fully charge. Moreover, there is no theoretical limit to a supercapacitor life cycle, whereas a lithium-ion secondary cell has a finite lifetime of about 500 cycles.
For all device types, the electrolyte properties determine the overall super capacitor terminal voltage. The voltage, when fully charged, is usually less than 3V. A conventional approach to constructing supercapacitors is comparable to that of a coin cell: lower and upper metal cases are joined by swaging to enclose the carbon electrodes and organic electrolyte.
With capabilities including high life cycle and fast charge and discharge times, small-cell supercapacitors can oust coin-type batteries from backup power duties in equipment ranging from IoT devices, smart meters, or medical devices to automotive electronics and industrial computing.
In automotive applications, the need for system backup during a power loss is becoming increasingly critical as connectivity and autonomous driving technology gain traction in modern vehicles. The automotive environment, however, is extraordinarily harsh with high temperature, humidity, and vibration. Traditional electrolytic capacitors and batteries will dry out over time under high-temperature conditions, resulting in increased series resistance and degradation of capacitance.
Specialized supercapacitors can be used in these situations to set design engineers free from the restrictions imposed by finite battery lifetimes. In addition, the supercapacitor’s benign open-circuit failure mode contrasts with typical short-circuit battery failures that may result in out gassing or ignition. As such, supercapacitors are the most cost-effective alternative to small backup batteries and can store enough energy to provide backup for durations ranging from a few seconds to several days, depending on the type of load and current demand.
The miniature supercapacitor from KEMET is an electric double-layer capacitor that uses a unique aqueous electrolyte solution that originated from NEC’s electric double layer capacitor, the first commercially available capacitor in the world in 1978. KEMET is the only producer of supercapacitors using aqueous electrolyte solutions in the world. Aqueous electrolytes are highly conductive, low environmental impact, non-toxic, and non-flammable, yielding strong performance and safety credentials. They also typically have greater resistance to moisture absorption than organic compounds, resulting in a longer lifetime with better stability.
The electrolyte of dilute sulfuric acid maintains high durability even under high temperature and high humidity conditions. A proprietary separator membrane has been developed from a high heat-resistant material whose pore diameter does not close even after extended use to improve the robustness at high temperatures. Small-cell super capacitors from KEMET feature a high-strength vulcanized rubber bond to ensure against liquid electrolyte leakage. The cross-section shown in figure 3 explains how these supercapacitors are constructed, including the aqueous electrolyte, rubberized electrodes, and separator membrane.
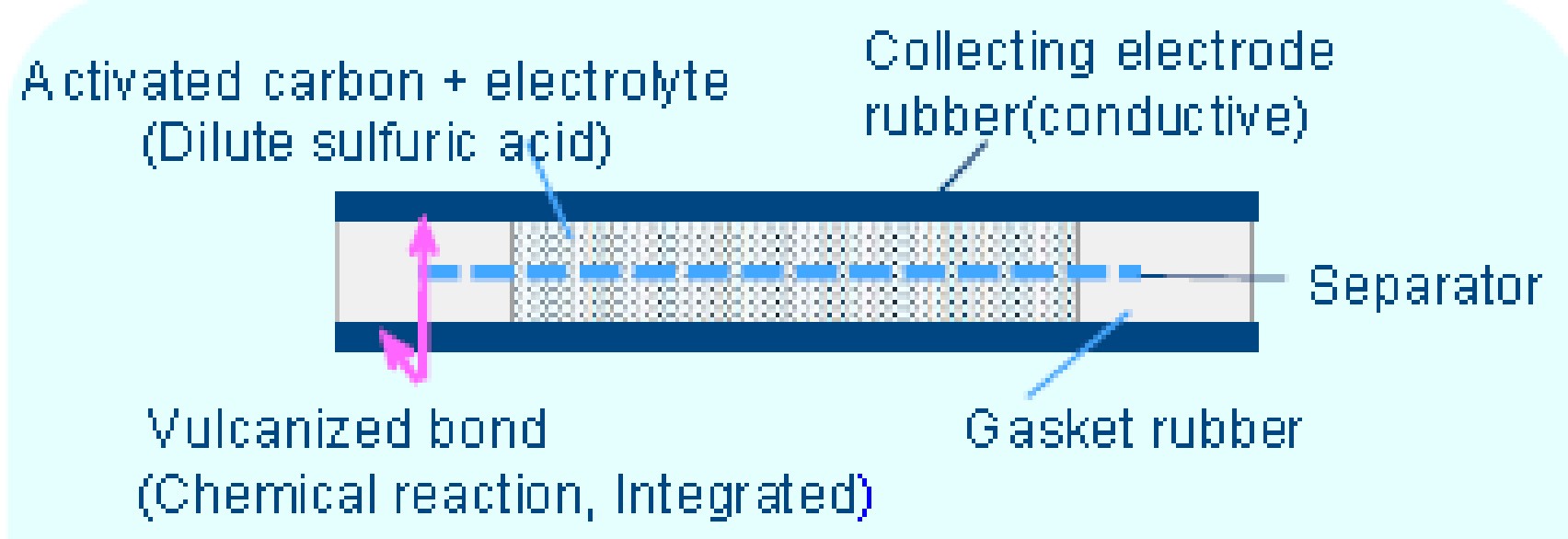
Figure 3: KEMET super capacitor construction
These features make KEMET supercapacitors an ideal choice for high reliability, harsh environments, including automotive applications. KEMET has released two supercapacitors that are qualified to an automotive testing protocol. These capacitors are manufactured in an ISO TS 16949 certified plant and are subjected to PPAP/PSW and change control. Following the March 2023 AEC-Q200 rev E issue, the SC Automotive Grade are now compliant with these requirements.
Figure 4 presents one such product, the FMD0H334ZF, which offers 0.33F and 5.5V. This high-capacity supercapacitor is built with a resin-molded package that enables tape-and-reel packaging and is compatible with automated mounting. It is the world’s first supercapacitor that is rated for 1,000 hours in a high temperature and high humidity environment at 85°C-85% and is also qualified to an automotive testing protocol for an operating temperature range of -40°C to 85°C.
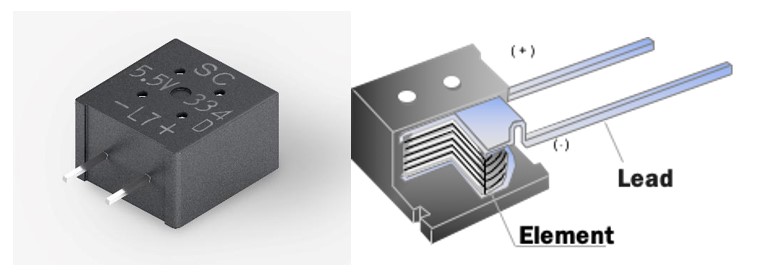
Figure 4: KEMET FMD0H334ZF supercapacitor and its construction
Figure 5 depicts another product, the FU0H105ZF supercapacitor, that offers 1F at 5.5V with the outer packaging built from a sealed metal can. This product is qualified to an automotive testing protocol and is a long-life device with operation exceeding 4,000 hours at 85°C. This harsh environment lifetime is equivalent to 10 years of life when referring to the mission profile near the dashboard of an automobile.
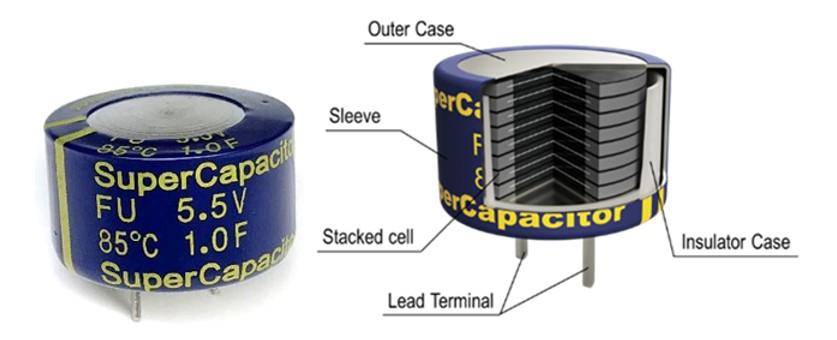
Figure 5: KEMET FU0H105ZF and its construction
The terminal material of these products is optimized to improve resistance to high humidity, thermal shock and vibration. The FU0H105ZF, in particular, has been carefully engineered for multi-stacking without increasing contact resistance due to a unique cell stacking technology.
Figure 6 also presents another product, the FMU0H334ZF Supercapacitor, that offers 0.33F at 5.5V. It is the world’s first supercapacitor that is rated for 1,000 hours at 105°C. Enhancements to the design and material upgrades with the same structure as FMD0H334ZF have been introduced to deliver 1,000 hours at 85°C/85% RH rated voltage and to meet automotive grade requirements with operational temperature range of -40 to 105°C.
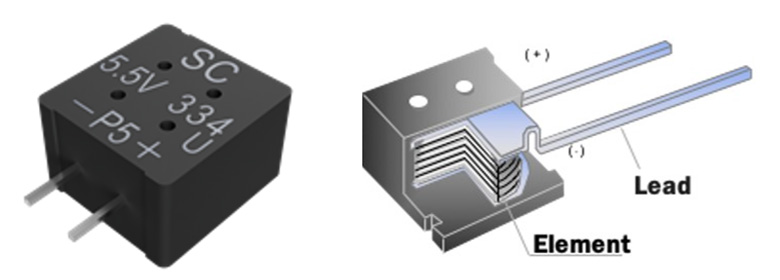
Figure 6: KEMET FMU0H334ZF Supercapacitor and its construction
Supercapacitors offer a high-performance alternative to batteries in many backup-power applications, delivering far greater cycle life and avoiding any need for designers to worry about battery replacement or recharging. The latest supercapacitors using KEMET aqueous electrolyte are cutting-edge energy storage devices featuring high voltage, long life, and environmental resistance required by the automotive market. KEMET new supercapacitors are ideal for use in automotive, medical, aerospace, industry, and other areas as required for high-reliability performance.