Polymer tantalum capacitors are emerging as an excellent choice for circuit designs that requires stable capacitance, long life, high reliability, and smaller size. Conductivity stability of Poly(3,4- ethylenedioxythiophene) (PEDOT) materials as well as capacitors with PEDOT cathodes were investigated in this study. PEDOT films and cathodes formed from insitu polymerization of 3,4- ethylenedioxythiophene monomer doped with p-toluenesulfonate (TOS) and pre-polymerized dispersion of PEDOT complexed with poly (styrene sulfonic acid) (PSSA) were compared under 85 °C/85% RH (relative humidity) environment. Electrical measurements along with Raman Spectroscopy, X-ray Photoelectron Spectroscopy (XPS), UV Spectroscopy, Thermo-mechanical Analysis (TMA), and Thermal Imaging were used to characterize the samples. Resistance of the films formed from insitu polymerization increased significantly on exposure to 85 °C /85% RH compared to the films formed from pre-polymerized dispersion. However, the trend was reversed in the capacitors where cathodes formed by insitu polymerization was more stable than the cathodes from PEDOT: PSSA. Our studies suggest that delamination caused by the thermomechanical and voltage induced stress generated in these capacitors is primarily responsible for conductivity degradation in polymer capacitors. These new insights led to the development of a new generation of PEDOT: PSSA polymer systems which significantly enhanced the reliability of polymer capacitors in harsh environments.
Conducting polymers have emerged from laboratory to mature commercial products in the last few decades and are now used in everyday applications ranging from mobile electronics to space electronics.1-3 Poly-(3,4-ethylenedioxythiophene) (PEDOT) is the most promising conducting polymer due to its high conductivity, high thermal stability, and commercial availability. Several commercial applications in electrical and optical devices have been developed utilizing the conducting properties of the in situ polymerized layers of the 3,4-ethylenedioxythiophene monomer doped with p-toluenesulfonate (TOS) molecules and PEDOT complex with polystyrene sulfonic acid (PSSA). One of the major application of these are in capacitors and PEDOT is the predominant counter electrode in both aluminum and tantalum polymer capacitors.4 Significant progress in development of conducting polymer based solid electrolytic capacitors has been made in recent years.
Application of intrinsically conducting polymer materials as the cathode for tantalum capacitors resulted in significantly lower Equivalent Series Resistance (ESR) when compared to MnO2 cathode systems.6,7 Higher performance and superior quality of polymer capacitors achieved over the last few years made these capacitors preferred components for many commercial applications. One of the drawback of polymer capacitors has been the reliability in high humidity environment. KEMET has developed number of technologies to solve this problem recently and were successful in developing polymer capacitors for high humidity applications.8,9 Young et al reported improved polymer capacitors with high reliability in 85 °C/85% RH (relative humidity) environment. Ye et al reported recently the successful introduction of polymer capacitors for automotive high humidity high temperature (85 °C/85%RH) applications.
Polymer capacitors are now increasingly being used for high reliability demanding applications in automotive, medical, military and space systems. A detailed understanding of mechanisms of the conductivity stability of these polymers in these environments is important for further development of these capacitors as well as their applications in more harsher environments. The loss of electrical conductivity of PEDOT on exposure to higher temperatures10-12 is mainly attributed to oxidation of PEDOT. Conductivity degradation under electrical bias13,14 has been reported in certain devices and the authors attributed this to de-doping of the conducting polymer. Young et al reported that extended exposure to 85 °C/85% RH under biased conditions results in ESR instability and they attributed this to “de-doping” of the conductive polymer. De-doping of the conducting polymer increases the resistivity of the counter electrode polymer and hence increasing dissipation factor and ESR.8 Peter et al reported that PEDOT and its conductive properties in solid capacitors decreases at elevated temperatures in the presence of humidity.15 They attributed this behavior of the polymer tantalum capacitors to the de-doping of the PEDOT under elevated temperature-humidity conditions. While these previous works suggests possible mechanisms such as de-doping for conductivity (or resistance) degradation in humidity environment, no supporting mechanistic studies were reported to explain the ESR degradation mechanism of polymer capacitors. In addition to the intrinsic conductivity stability of these materials, their interfacial resistance performance in multilayer cathode systems is critical for the successful performance of these components in harsher environments. The objective of this study is to investigate the mechanisms of intrinsic and interfacial conductivity (or resistance) changes on humidity exposure for insitu PEDOT (PEDOT: TOS) and PEDOT: PSSA and capacitors based on these materials.
PEDOT Film Fabrication. In-situ oxidative polymerization of PEDOT was performed by polymerization of 3,4 ethylenedioxythiophene (CleviosTM M V2 from Heraeus) in the presence of iron (III) toluenesulfonate (CleviosTM C-E from Heraeus) with a monomer/dopant ratio of 3:1. The in-situ film was fabricated as follows: A glass substrate was immersed in oxidizer solution, then taken out and dried for 30 minutes in ambient conditions. This was followed by dipping in monomer. Polymerization was completed by drying for one hour. The polymer film on the glass substrate was washed sequentially with ethanol and deionized water. The obtained film is in a doped state with tosylate ion (TOS) as counter-ion. The film from the pre-polymerized polymer was prepared by casting the as received CleviosTM K V2 HV (Heraeus) (PEDOT: PSSA and additives) dispersion on a glass substrate and subsequent drying at 130 °C for 30 min.
Capacitor Fabrication with PEDOT Cathode. Tantalum powder was pressed into rectangular pellets of 2.5 mm × 2.5 mm × 4 mm width and 6.5 g/cm3 density. The pellets were sintered in vacuum at 1350 °C for 20 min. Tantalum anodes were anodized in an aqueous solution of 0.1% of phosphoric acid at 80 °C. In-situ PEDOT (PEDOT: TOS) cathodes were prepared by oxidative polymerization of PEDOT on the anode by polymerization of 3,4 ethylenedioxythiophene (CleviosTM M V2 from Heraeus) in the presence of iron (III) toluenesulfonate (CleviosTM C-E from Heraeus) with a monomer/dopant ratio of 3:1. The anodes were dipped in the oxidizer CE followed by dipping in monomer MV2, drying and washing. This process was repeated a few times to build PEDOT film inside the anode pores and the surface of the anode. Pre-polymerized (PEDOT: PSSA) cathode were fabricated by dipping the anodes in the prepolymer dispersion CleviosTM K V2 HV multiple times to build the external layer on the surface of the anode. Conductive carbon particle filled layer and conductive silver particle layers were applied over the PEDOT layer followed by assembling onto lead frame using conductive adhesive. These assembled capacitors were molded using phenolic epoxy molding compound. The molded capacitors were mounted to the PC board using a lead-free solder reflow process (260 °C peak temperature).
Film Characterization. The sheet resistance of the films was measured by a four-point probe with a Keithley 2400 source meter. Raman spectra was recorded on a Ramnor HG2S spectrometer (Jobin-Yvon) with Ar laser (514.5 nm) at a resolution of 0.5 cm−1 . XPS of the films was performed using a Shimadzu Axis Ultra DLD spectrometer. TMA were performed using a Perkin Elmer TMA. UV Spectra was recorded using Perkin Elmer UV Spectrophotometer. Joule heating studies were conducted using thermal imaging with an IR Camera coupled with current and voltage source.
Capacitor Characterization. ESR of the capacitors were measured at 100 kHz using Agilent E4980A Precision LCR Meter.
Intrinsic Resistance Stability
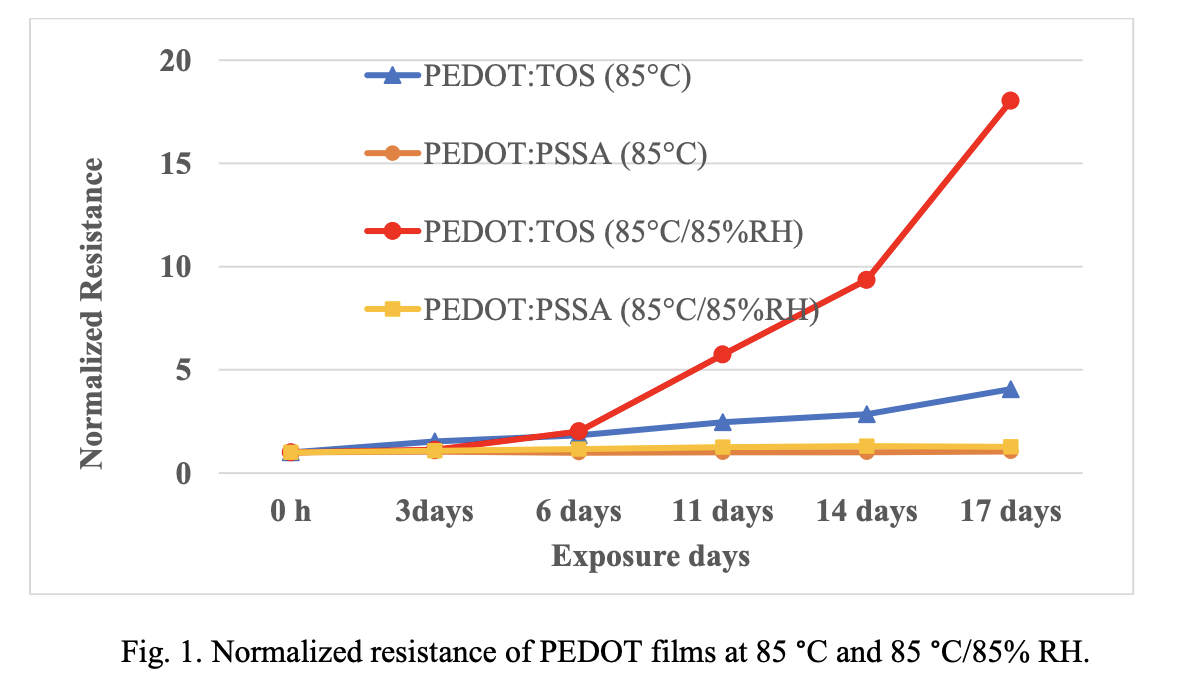
Films of both PEDOT: TOS and PEDOT: PSSA were exposed to 85 °C/85% RH humidity chamber and the resistance changes were measured periodically. Fig.1 shows normalized resistance change of PEDOT: TOS vs PEDOT: PSSA as a function of exposure days. After exposure to 14 days at 85 °C/85% RH, resistance of the PEDOT: TOS film showed a larger increase compared to that of PEDOT: PSSA. Films were also exposed to 85 °C to differentiate the effect of temperature and moisture on the resistance change. Results suggests that humidity exposure has a greater influence on the resistance degradation compare to the temperature. Resistance of the PEDOT: PSSA was practically unchanged during the tests at 85 °C and 85 °C/85% RH.
This large change in resistance of the PEDOT: TOS film relative to PEDOT: PSSA can be explained by the morphological differences in these materials. Molecular structural studies of PEDOT: TOS show a structure that comprises crystallites (aggregates of several π−π stacked PEDOT chains) embedded in an amorphous matrix of PEDOT chains.16 The crystallites are linked by interpenetrating π−π stacked chains such that percolative paths are formed in the structure. In the computational study, the authors observed that in the dry phase PEDOT chains and crystallites are situated closer to each other as compared to the wet phase (mixture of water molecules and PEDOT chains). This indicates that the presence of water molecules increases the distance between these PEDOT crystallites. It is possible that the higher concentration of water at high humidity conditions can increase the distance between these crystallites to a greater degree and this can increase the percolative paths causing an increase in resistance. Raman Spectroscopy and X-ray photoelectron spectroscopy were used to investigate the structural changes on humidity exposure.
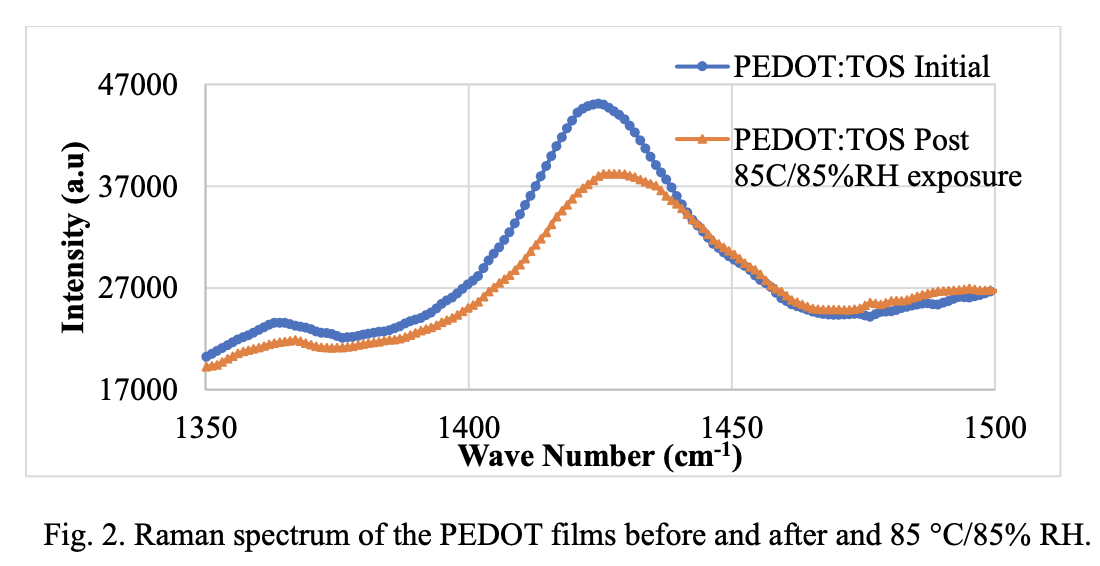
Raman Spectroscopy. The Raman spectrum of the pristine PEDOT: TOS films and the films exposed to 85 °C/85% RH for 20 days are shown in Fig. 2. The Raman spectrum exhibits the characteristic peak of PEDOT including C-C stretch at 1366 cm-1 and the symmetric C= C(-O) stretch at 1424 cm-1 1 . The strong symmetric C= C(-O) stretch at 1424 cm-1 band of PEDOT assigned to the ring C=C stretching vibration. On exposure to humidity conditions, the band at 1424 cm-1 is shifted upwards to 1426 cm-1. We also observed a decrease in intensity of this peak. The small upward shift and the decrease in intensity of this peak suggest that a small decrease in conjugation. This decrease in conjugation is probably due to a decrease in effective conjugation from the chain segments because of morphological changes discussed in the earlier session.
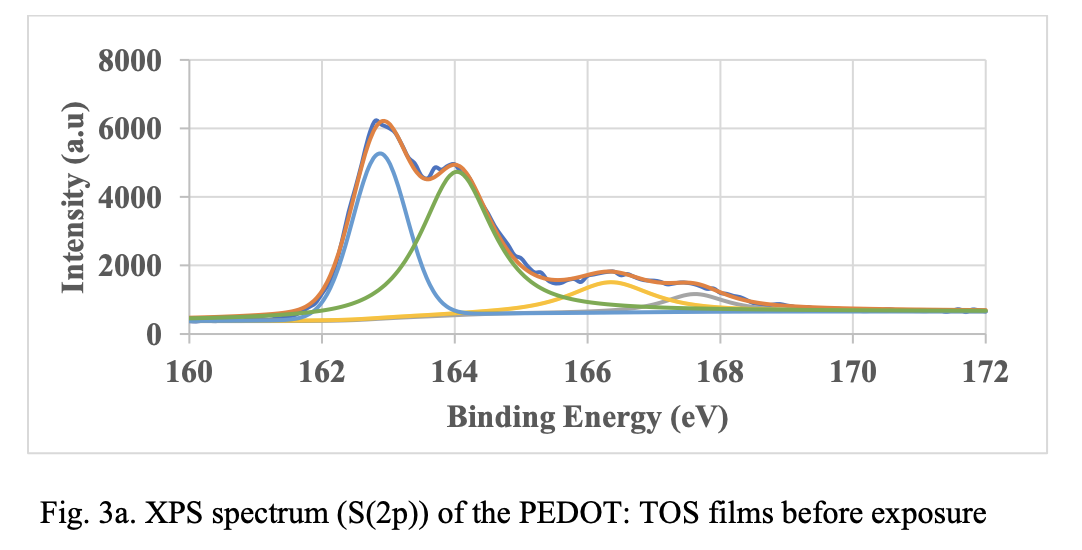
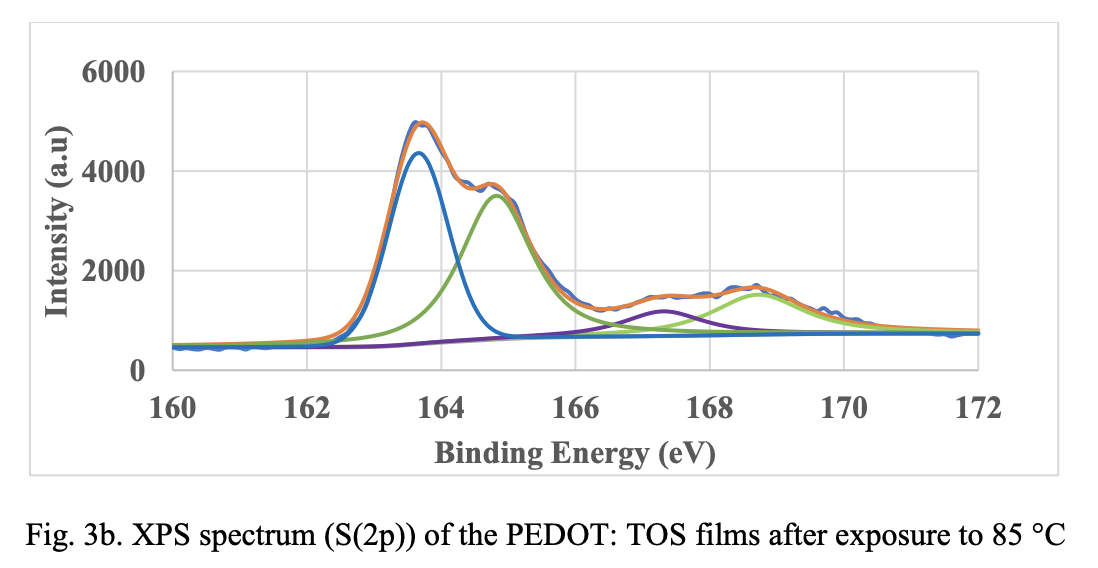
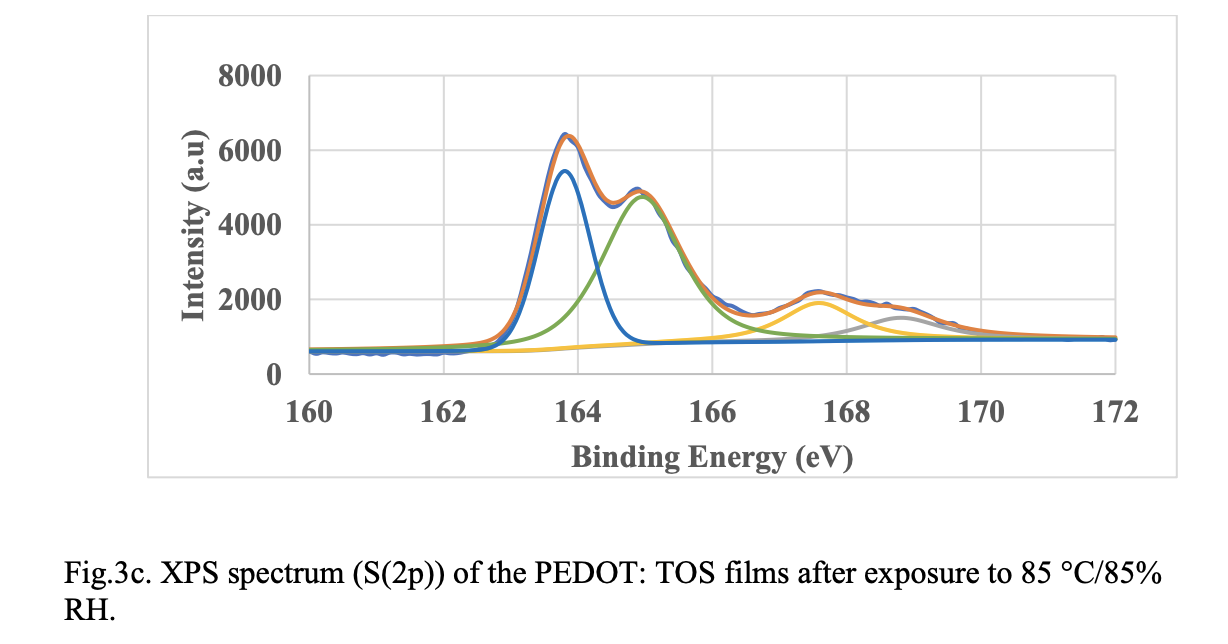
XPS Spectroscopy. Fig. 3 shows deconvoluted S(2p) XPS spectra of in-situ PEDOT material before and after exposure to 85 °C and 85 °/85% RH. The binding energy peaks at 162.6 and 163.8 eV are assigned to spin-split doublets of sulfur atom from PEDOT. As the films are exposed 85°C and 85 °C/85% RH, peaks at 162.6 and 163.8 shifted to higher binding energy indicating oxidation of the polymer. However, the peak shift to higher binding energy is not significant when comparing the XPS spectra at 85 °C and 85 °C/85% RH. This suggests that oxidation alone cannot explain the larger resistance change seen on humidity exposure. As mentioned before morphological changes and resulting changes in percolation pathways could be contributing to the resistance change.
Structural studies by Xing et al. shows that the main difference between PEDOT doped with two different anions is the amount of dopant localized on the surface.17 XPS studies suggest that dopant amount on these surface is about 25% for TOS- and about 50% for PSS- . The large high molecular weight anion (PSS- ) segregated at the surface of the PEDOT: PSSA film is possibly acting as a barrier to prevent moisture intrusion into the PEDOT grains. Enhanced thermal stability of PEDOT: PSSA is also reported to be attributed to the protective effect of PSSA shell around PEDOT.
Interface Resistance Stability
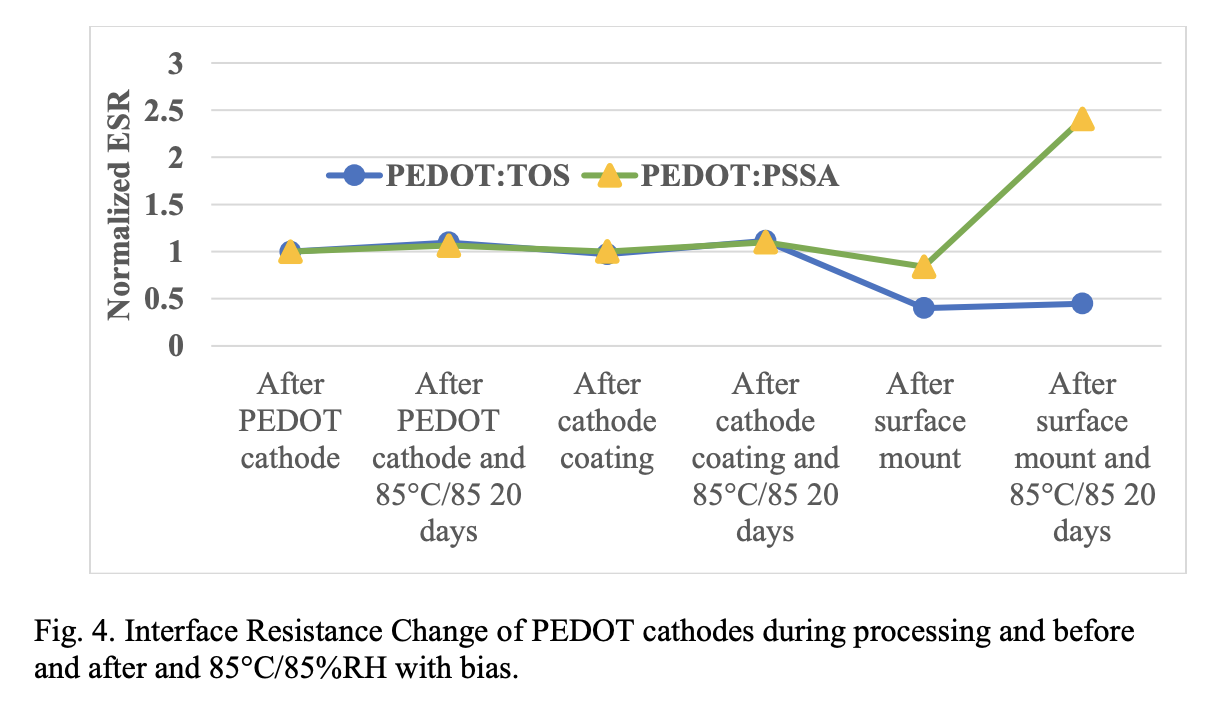
Fig. 4 shows normalized ESR change of the capacitors prepared with PEDOT: TOS and PEDOT: PSSA cathodes when exposed to biased 85 °C/85% RH conditions. The ESR of a capacitor is the internal resistance which includes the resistance of the primary cathode layer (PEDOT) and the cathode coating layers (carbon or silver particle filled coating) that appear in series with the capacitance of the device. Cathode coating layers such as carbon filled polymer layer and silver filled polymers are very stable to humidity conditions and their contribution to resistance change is considered negligible. Capacitors were exposed to humidity conditions after each of the processing stage and the effect on the resistance was monitored. No significant changes in resistance was observed for the parts exposed to 85 °C/85% RH after PEDOT cathode and after cathode coating application for both cathode systems. However, the resistance of PEDOT: PSSA cathode increases after surface mounting and 85 °C/85% RH exposure compared to PEDOT: TOS cathode. This suggests that the thermomechanical stresses during reflow/mounting conditions (260 °C) due to coefficient of thermal expansion (CTE) mismatch caused weakening of the interfaces. CTE of the cathode layer is an order of magnitude higher than that of the tantalum substrate. During surface mounting, a relatively higher stress proportional to the difference in CTE and the change in temperature is generated at the tantalum polymer interface. If the adhesion of the PEDOT layer is lower than this stress, interfacial delamination can occur and cause an increase in ESR. The higher resistance stability of the PEDOT: TOS cathode is due to the higher adhesion of the PEDOT: TOS to the substrate due to in-situ polymerization.
On exposure to bias 85 °C/85% RH, the resistance further increases due to the hygroscopic stress (moisture induced stress) generated during moisture absorption and differential swelling of the interfaces. Hygroscopic stress contributes to the formation and enlargement of moisture pathways in the coating. Hygroscopic stress is proportional to the coefficient of moisture expansion of the PEDOT layer since the tantalum substrate has negligible moisture expansion. The PSSA part of PEDOT: PSSA is strongly hygroscopic and this causes higher moisture sorption and hence higher coefficient of moisture expansion. A higher moisture induced stress is thus generated in the PEDOT: PSSA /tantalum interface than PEDOT: TOS /tantalum interface.
We studied 85 °C /85% RH polymer capacitors both at unbiased and biased 85 °C/85% RH conditions. Our studies show that a relatively larger increase in ESR change was observed when these capacitors were tested in biased 85 °C/85% RH conditions. This suggest that the application voltage has some role in causing this increased ESR change and the effect of voltage has a larger contribution than those due to thermomechanical and hygroscopic stress. We propose that in addition to the thermomechanical stress and moisture induced stress, voltage induced stress also contributes to the interfacial delamination. Conducting polymers are reported to undergo dimensional changes under application of voltage and this property is used in actuators. Okuzaki reported PEDOT: PSSA films contracts on application of electric field and generate contractile stress proportional to the voltage applied.17 We further investigated whether the bias application during humidity exposure can induce dimensional changes in the PEDOT cathode layers and its effect on the interfacial film integrity.
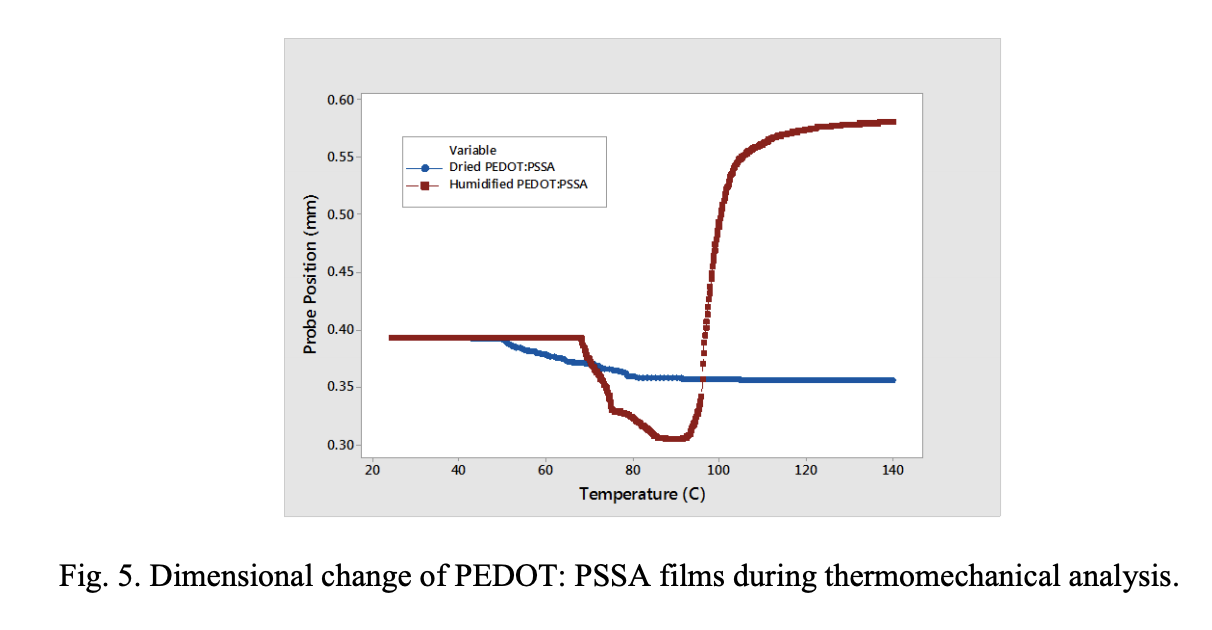
Thermomechanical Studies. TMA studies were conducted on the PEDOT: PSSA film to investigate the effect of temperature and moisture content in the film on dimensional changes. Fig. 5 compared the dimensional changes in a completely dried film and humidified PEDOT: PSSA films. We observe contraction of the film as moisture evaporates during temperature rise. This contraction increases with the amount of moisture content in the film. Increases in temperature causes these polymer films to expand. Such contraction and expansion can generate stresses in the film and at the interface.18 As seen in Fig.5, we observed a relatively large expansion of the humidified PEDOT: PSSA film immediately following the contraction. This expansion is due to the lifting of the film from the substrate caused by the stress generated at the interface. We hypothesize that a similar mechanism is happening in the capacitor cathode layers at 85 °C/ 85% RH humidity conditions causing weakening and delamination of the interfaces.
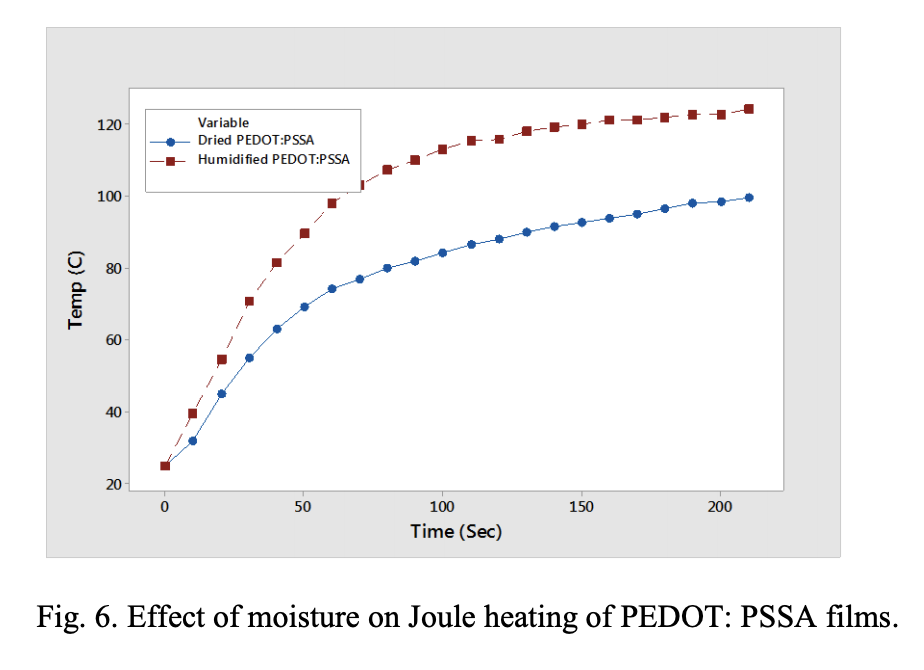
Thermal Imaging and Joule Heating. Thermal imaging of the films was monitored with applied voltage to see the Joule heating in these films. Fig. 6 shows the temperature rise when voltage (10 V) is applied to PEDOT: PSSA film. The temperature in the film increases due to Joule heating which is proportional to the current applied and the resistance of the film. Note that the temperature rise is higher for humidified film due to the higher resistance of the moisture sorbed conducting polymer film compared to the dried conducting polymer film. Application of bias (voltage) increases the local temperature in the polymer cathode and this increases with increased humidity in the environment. It can be theorized that the local temperature of parts exposed to 85 °C/85% RH is higher than 85 °C due to this observed Joule heating effect. A higher expansion due to this increased temperature can thus be expected during bias testing. As discussed before, the stresses generated from this contraction and expansion is one possible reason for higher ESR increase when polymer capacitors are exposed to bias humidity conditions.
Dedoping. Another possible effect of voltage is the dedoping of conducting polymers. We have investigated the potential effect of dedoping in the resistance stability of the PEDOT films. Fig. 7 shows the schematic of the experimental set up to demonstrate the dedoping of the conducting polymer films when subjected to a voltage. The PEDOT: PSSA films were separated with a gap filled with moisture. A gap between the polymer films was introduced to simulate the delamination. When voltage (10 V) was applied, resistance at the negative electrode increased from 5.11 ohms to 25.8 ohms whereas the resistance at the positive electrode increased from 5.11 to 100 kohms. At the negative electrode, polymer film gets reduced and at the positive electrode, polymer film overoxidized and became highly resistive. No change in resistance was observed when the gap was not present in the film. This suggests that a delamination is a prerequisite to de-doping.
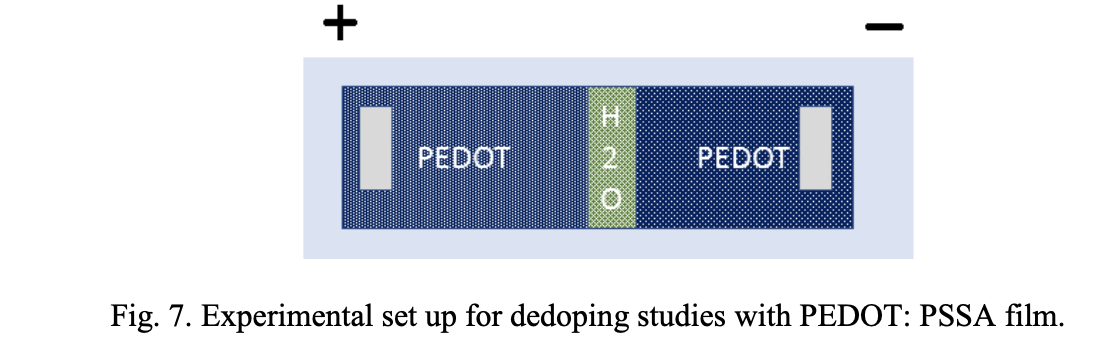
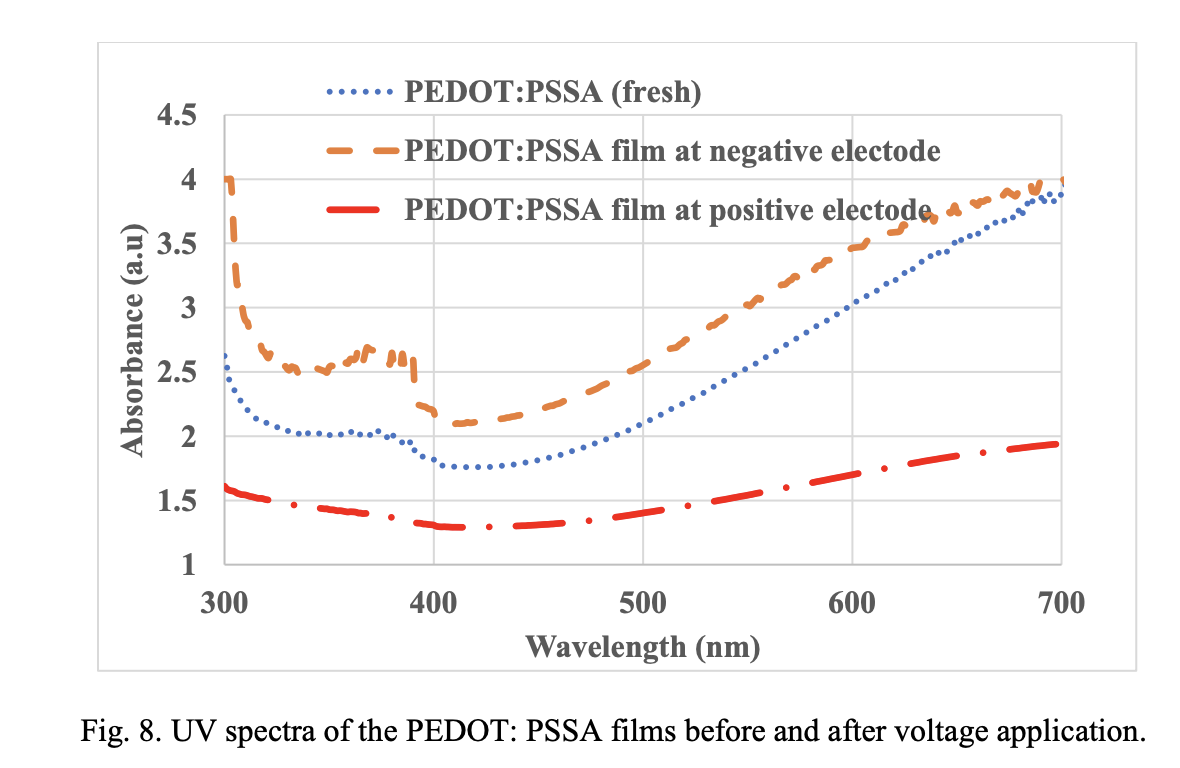
The reduced and overoxidized state of this film was confirmed by UV spectroscopy (Fig. 8). The pristine film was weakly absorbing and shows the expected polaronic tail beyond 600 nm. This polaronic tail is absent in the film from the positive electrode indicating the high resistance nature of this film.
Humidity Degradation Mechanism
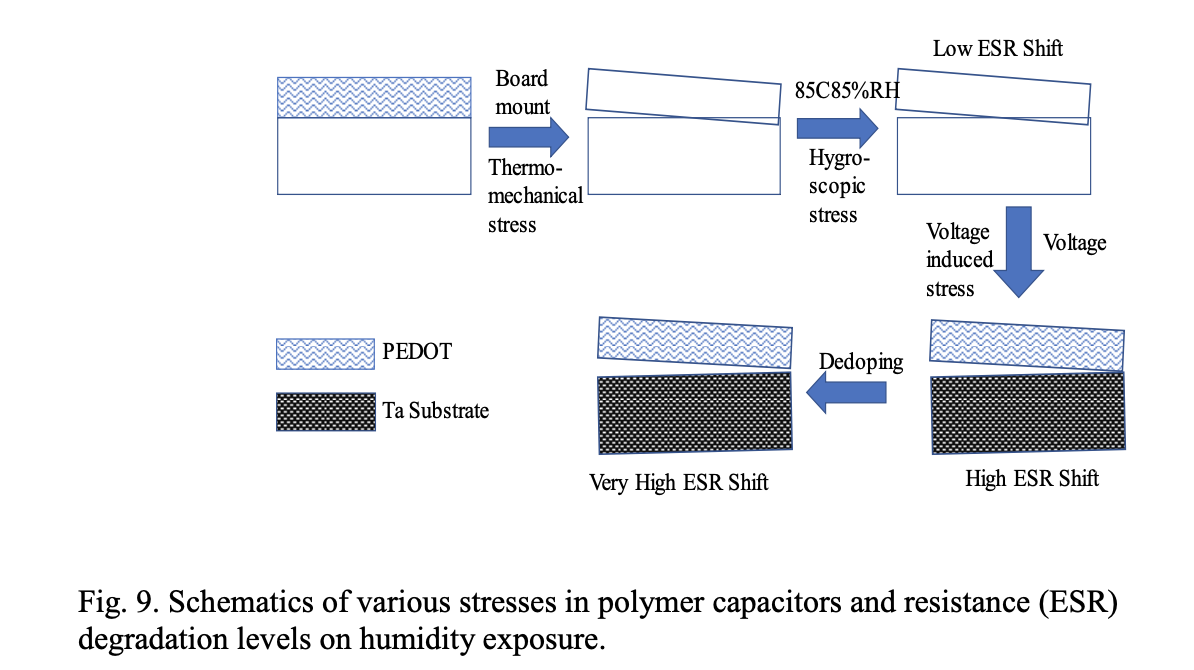
Our studies suggest that the interfacial resistance degradation of the PEDOT cathode layers is responsible for the ESR degradation in polymer capacitors. Interfacial resistance is degraded by delamination of the conducting polymer layers caused by a combination of thermomechanical stress, hygroscopic stress, and voltage induced stress. Delamination is thus the primary reason for humidity degradation in polymer capacitors. The degree of ESR degradation depends on the moisture sorption of the conducting polymer and the adhesion of the polymer to the substrate. When polymer layers are significantly delaminated, polymer de-doping can occur which can further degrade the ESR of the capacitor. In the absence of significant delamination, dedoping is difficult. This leads to the suggestion that delamination followed by de-doping is the primary mechanism of ESR degradation (ESR shift) when polymer capacitors are exposed to high humidity conditions.
We propose following three levels of resistance degradation in polymer capacitors on humidity exposure
a. Thermomechanical stress + hygroscopic stress → low ESR shift
b. Thermomechanical stress + hygroscopic stress+ voltage induced stress → high ESR shift
c. Thermomechanical stress + hygroscopic stress+ voltage induced stress + dedoping → very high ESR shift
New Conducting Polymers
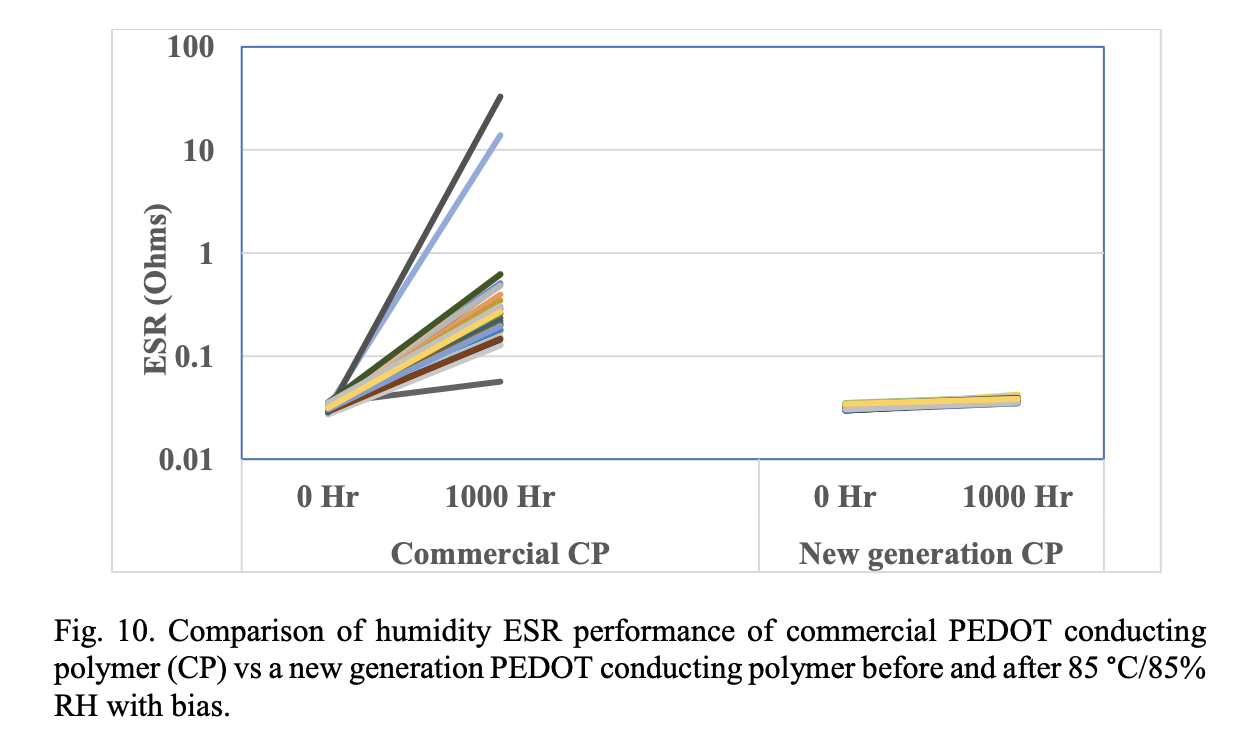
The two critical material factors causing delamination of the PEDOT: PSSA systems are the adhesion and moisture sorption properties of the polymer. We have developed a new series of conducting polymers19 which showed improved adhesion and lower moisture sorption properties than current commercially available PEDOT: PSSA materials. Fig. 10 shows a comparison of the ESR reliability of a commercially available conducting polymer based polymer capacitor and a new generation of conducting polymer based polymer capacitor. New generation of conducting polymers showed superior ESR stability in 85 °C/85% RH biased environmental conditions.
The electrical properties of the conducting polymer films and conductive polymer based capacitors on exposure to high humidity conditions were investigated in this study. The resistance of the PEDOT: TOS films increased significantly on exposure to 85 °C/85% RH whereas the PEDOT: PSSA films were very stable. A different trend was observed in the capacitors. Capacitors with PEDOT: PSSA cathodes showed higher resistance on exposure to humidity. ESR stability at various stages of the capacitor fabrication suggests that thermomechanical stresses cause weakening of the interface. Humidity stresses and voltage stress further accelerate this interface weakening leading to delamination and higher resistance. Thermomechanical studies and thermal imaging studies support this delamination mechanism. Our studies also suggest that de-doping can take place on voltage application when the conducting polymer layers are delaminated and the layers are separated by moisture at the interface. These studies suggest that PEDOT: PSS polymer with enhanced adhesion, lower moisture sorption and robust to these stresses are needed for high reliability applications. Our investigations led to a new generation PEDOT polymer cathode materials with improved reliability under high temperature high humidity conditions.
Antony P. Chacko, Yang Jin, Yaru Shi, Ajaykumar Bunha, James Chen, and Philip M. Lessner